Our world is facing a COVID-19 pandemic. It has claimed many lives in different parts of the globe and many health experts have worked together to respond to this crisis. Developing a vaccine to fight against the virus is critical at this point. According to the World Health Organization (WHO), there were more than 200 vaccine candidates that were in development, and among them, 60 were in clinical development, meaning, it has undergone a three-phase process.
During Phase 1, the vaccine is in trial with a small group of infected patients. In Phase II, the study expanded. The vaccine is given to people of distinct ages and physical health to evaluate the results of the test. In Phase III, the vaccine is used on a large scale of people to test its security and its effectiveness. And lastly, in Phase IV, the vaccine is approved and licensed.
Read: Perks of the Vaccinated
As Bria house and lot owners and potential home buyers, we want to keep you safe within the community amidst the pandemic, so here are some of the frequently asked questions (FAQs) that can help you understand the COVID-19 vaccines.
When will the COVID-19 vaccines be available to me?
The vaccines available in the Philippines are Sinovac and AstraZeneca and currently, the government is in its initial phase of rolling out its vaccination, partnering with the local government units.
Your local government unit will arrange for the registration and scheduling of vaccination so it would be best to coordinate with your city health office for the availability of vaccines.
Who can take the Covid-19 vaccine?
Based on the World Health Organization (WHO) Strategic Advisory Group of Experts (SAGE) on Immunization, it follows a COVID-19 prioritization framework. This structure was formulated due to the limited global supply of COVID-19 vaccines.
The Philippine government implements strategies in cooperation with the local government unit executing the vaccination plan to guarantee the fair dispersal of vaccines for all Filipinos.
In the vaccine distribution plan, part of the prioritization frameworks were senior citizens, frontline health workers, uniformed personnel, and indigent population. Having a prioritization framework reduces mortality and conserves the health system capacity of the Philippines.
There were three groups in the priority population for the COVID-19 vaccination:
The priority population of the widespread vaccination are Frontline workers in national and local health facilities, health professionals in public and private and those who are in the health allied professions, nurses, janitors, barangay health workers belong to Group A. Also in this group were senior citizens, adults with comorbidities.
Meanwhile, in Group B, these are teachers, social workers, government workers, OFWs, and socio-demographic groups and, another remaining workforce. Those who belong in Group C are the rest of the Filipino population not part of the former groups.
Am I eligible to receive the Covid-19 vaccine?
The eligibility to receive the vaccine depends on the person’s health status. You are qualified to get the vaccine but with specific protections. If you have a history of bleeding disorders, or with food allergies (egg or medicine) and with a history of asthma. Coordinate with your local government health workers for your schedule.
You are also eligible but for rescheduling if:
• You are currently diagnosed with COVID-19 or you have the symptoms such as fever, coughs, colds, chills, fatigue, headache, sore throat, loss of taste and smell, difficulty in breathing.
• You have antibodies for COVID-19 in the past 90 days.
• You are having a pregnancy in the first 3 months.
• You received a vaccine in the past 14 days
• You have been admitted due to co-morbidities in the last 3 months.
• Your blood pressure is 180/120 or hypertensive.
Also, before your Covid-19 vaccination, you must secure a medical clearance from an attending physician or primary health care provider if:
• You have an autoimmune disease.
• You were diagnosed with Human Immunodeficiency Virus
• You underwent a transplant.
• You are currently on steroids.
• You are a cancer patient who is undergoing chemotherapy treatment.
• You are in poor health status with a life expectancy of less than six (6) months
On the other hand, you are not eligible if:
• You have allergies in vaccine components
• You are less than 18 years old
• You experience a severe allergic reaction after the first dose of the vaccine
Some people have underlying medical conditions such as the following:
Is the Covid-19 vaccine safe? Do they work?
Vaccines with the approval of Emergency Use Authorization by the Food and Drug Administration (FDA) are effective and safe based on the studies provided by the experts.
Vaccination is important to keep yourself from getting COVID-19. Vaccines cannot make you sick with COVID-19. Also, please make sure to follow the minimum public health protocols (Wear a face mask, face shields, social distance, and sanitize hands)
How do vaccines work?
Vaccines vary in their main components on how they can trigger the immune system in creating antibodies. Vaccines imitate the virus that causes the infection in one’s body. Antibodies protect a person that is infected with the virus.
Vaccines can damage or inactivate the copies of the whole or portion of the virus and make protein copies.
The World Health Organization expresses the effectiveness of the vaccines as it reduces the infection in the vaccinated group through the clinical trial.
The immunity from the vaccine is currently unknown, this is being determined through thorough monitoring and trials from the vaccinated group. Until such time that the immunity is established, it is best to always practice health protocols.
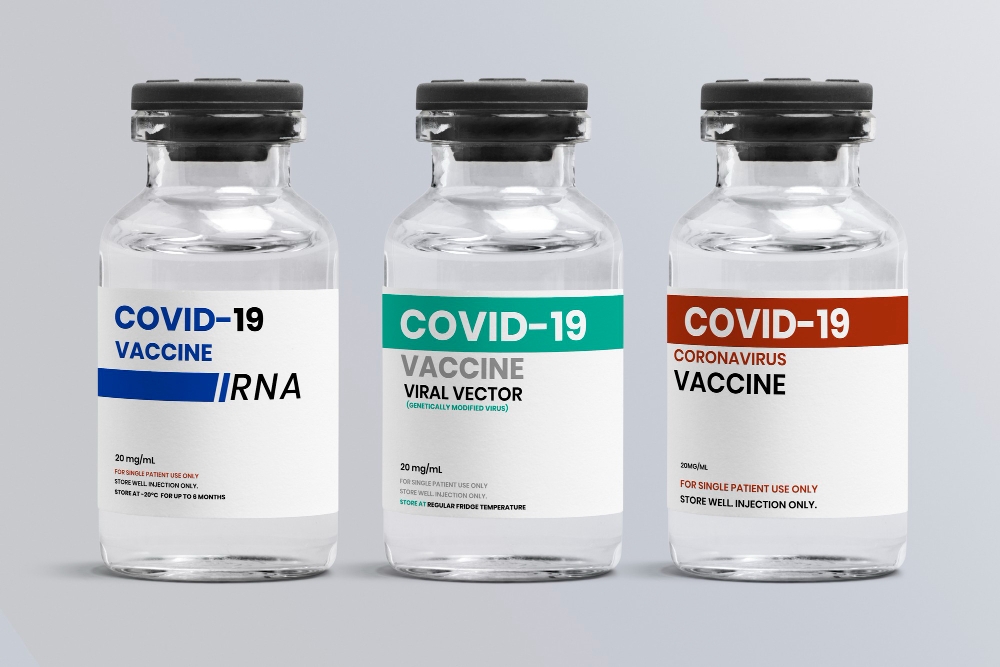
The Different Types of Covid-19 Vaccines:
1. Sinovac
It is a biopharmaceutical company based in Beijing. It works by using the killed viral particles to expose the body’s immune system to the virus. Based on this BBC article entitled “Covid: What do we know about China’s coronavirus vaccines?”, Sinovac’s main advantage is that it can be kept or stored in a standard refrigerator at 2-8 degrees Celsius.
Sinovac is more beneficial in developing countries as it may store the vaccine doses in low temperatures (2-8 degrees Celsius)
What are the possible side effects?
The same with any other vaccines available, you may experience side effects after taking the COVID-19 virus.
These are the common side effects:
Feeling unwell
• Lethargic or feeling fatigued
• Chills or feverish
• Headache
• Tenderness, pain, redness, itching, or swelling of the arm
• Muscle pain
If you feel these side effects, generally, your body is building protection against COVID-19. Usually, the symptoms go away on their own for 1 – 3 days. If these do not go away after a few days, consult your nearest healthcare professional.
So, what to do next? If you experience headache, muscle pain, and fever, take ibuprofen it will help you ease the pain. But if you have to swell on the injection site, use a cold compress so the swelling can subside.
2. Pfizer
According to the website of the Centers for Disease Control and Prevention, Pfizer has not been approved or licensed by the U. S Food and Drug Administration but was granted with the Emergency Use Authorization (EUA). Pfizer was found 95 percent effective upon the large-scale clinical sample administering of the Pfizer – BioNtech vaccine.
What are the possible side effects?
Reactogenicity symptoms were common but mostly mild to moderate.
You may feel and these side effects:
Feeling unwell
• Lethargic or feeling fatigued
• Chills or feverish
• Headache
• Tenderness, pain, redness, itching, or swelling of the arm.
• Muscle pain
The Centers for Disease Control and Prevention will continue to work and provide updates on how well the Pfizer- BioNtech vaccine works in different conditions.
3. AstraZeneca
According to this website, the Food and Drug Administration issued emergency use authorization (EUA) for AstraZeneca on January 28, 2021. Therefore, it can be used to inject vaccines among the 10 percent population in the Philippines.
The World Health Organization listed the two versions of AstraZeneca for emergency use – Republic of Korea (AstraZeneca-SKBio) and the other one is the Serum Institute of India. Both underwent review by the European Medicines Agency. (EMA)
The EMA is strictly assessed based on the safety, effectiveness, and quality of the vaccines.
How will I get COVID-19 AstraZeneca Vaccine?
Two (2) doses will be given to you, COVID-19 Vaccine AstraZeneca will be injected into your upper arm muscle. Make sure to get an appointment with your local health center and complete the vaccination series.
Meanwhile, in this article, some countries in the European Union have suspended the use of AstraZeneca for precautionary measures. The person who received the vaccine has experienced blood coagulation disorders.
Is AstraZeneca safe?
In the study made in the University of Oxford for the AZD1222, the analysis was from the UK and Brazil and the vaccine proved efficacy up to 90%. The vaccine is effective in avoiding the COVID-19 virus with no hospitalization or severe cases.
The efficacy of the vaccine is at 70%, combining the analysis from the trials in the UK and Brazil.
4. Moderna
Based on the website of the Centers for the Disease Control and Prevention, Moderna is like Pfizer, the vaccine type is mRNA. It requires 2 shots one month apart. The vaccine should be shot in the upper arm muscle. It was authorized by the Food and Drugs Administration in December 19. It’s efficacy rate is 94.5%. The vaccine is stable at 36-46 Fahrenheit temperature, more like the temperature of a standard medical refrigerator. It is at 100% preventing hospitalization and death. It is also effective against the UK variant and South African variants.
What are the possible side effects of Covid-19 Modern vaccine?
Once the shot is done, you may experience:
- Pain
- Redness
- Swelling
Throughout the rest of your body:
- Tiredness
- Headache
- Muscle pain
- Chills
- Fever
- Nausea
You may experience these side effects but they should go away in a few days. In this article, the Philippines has now secured 20 million doses of COVID-19 Vaccine Moderna. To keep you safe within the Bria Homes Community, it would be best to contact the local health officer nearest you.
5. Sputnik V. and Janssen
Both vaccines were developed by Russia’s Gamaleya National Center of Epidemiology and Microbiology.
Johnson’s & Johnson’s Janssen is a vaccine that uses a common cold virus as a host. Janssen vaccine carry part of the COVID-19 genetic order into the body. This informs the body that there is a coronavirus without risking the patients’ lives.
Is Sputnik V. and Janssen Covid-19 vaccine effective?
In the clinical trial in the U.S, it was tested at 72 percent effective at stopping infections.
How to store the Covid-19 vaccine?
The vaccine can be in store at a regular refrigerator and can give you at 66% level of protection. This has the potential of a faster mobilization of the vaccine around the world. Sputnik V’s storage is just a standard fridge. Meanwhile, the Sputnik-V vaccine utilizes an inactivated virus, just like the Oxford and Sinovac vaccines to stimulate an immune response from the body.
How effective is it?
Several testing showed that 90 percent effective in individuals aged over 60 years old. Further testing will be made to ensure the 100% efficacy of the vaccine Sputnik V and Janssen
6. Covaxin
It is a vaccine developed in India – Bharat Biotech, a 24- year- old vaccine maker. Once Covaxin is administered, the immune cells can still recognize the dead virus, signaling the body to make more antibodies to fight off the coronavirus.
Covaxin had become a controversial vaccine due that the vaccine was administered even if the study is incomplete. It was used even without the approval to use the vaccine in emergency situations.
It was defended by the All India Drug Action Network and the manufacturer that Covaxin is safe and provides intense immune response.
Aftercare once vaccinated.
It is our responsibility to protect ourselves and to protect our family. Always practice the minimum public health protocols.
- Avoid crowded places
- Wear mask and face shields
- Maintain social distancing at least 1 meter
- Wash/ Sanitize hands
- And observe proper ventilation at home and workplace
Amidst the situation we are facing right now, we, in Bria Homes Community would like to make sure that you are all responsible in your own health and welfare. Always take care of yourself and read and understand each vaccine before registering or scheduling your name.
BRIA Homes is a subsidiary of GOLDEN MVHoldings, Inc., one of the largest real estate companies in the country. BRIA Homes is primed to bring quality andaffordable house and lot packagesand condominium units closer to ordinary Filipino families. This is the goal that drives every single employee in the company, for which the ultimate fulfillment is seeing a client happily moving into BRIA’s homes.
To know more, visit their website at www.bria.com.ph, like and follow “Bria Homes, Inc.” on Facebook, Twitter, Instagram, YouTube, Pinterest, Spotify, Viber Community, Telegram Channel, Kakao Talk, LINE and WhatsApp, or call 0939-887-9637.